|
COURSE LECTURE NOTES:
|
SCIENCE | MATTER |
SOLAR SYSTEM |
PLANETS | ATMOSPHERE |
WIND and TEMPERATURE |
HUMIDITY | WEATHERING |
SOIL |
SEASONS | MASS WASTING |
SEASONS and CLIMATE |
WIND WORK |
STREAMS | LAND FORMS |
GROUND WATER |
CAVES/KARST | THE OCEAN |
TIDES & ESTUARIES |
WAVES | GLACIERS |
GLACIAL LANDFORMS |
VOLCANOES | VOLCANOES |
CHON | PLATE TECHTONICS |
EARTHQUAKES |
ROCKS |
CLIMATE CHANGE |
MAKE UP OF MATTER
Element = simplest form of matter, cannot be broken into anything simpler by normal means
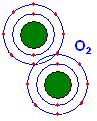
Atom = smallest part of a element, it can be broken into smaller pieces by fisson(abnormal means).
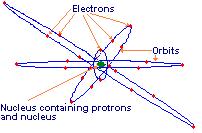
Everything is made up of atoms. Parts of an atom are:
Proton – located in the nucleus of the atom, it has an atomic mass unit (amu) weight of 1, it has a positive (+) electrical charge of 1. The number of protons = the Atomic Number
Neutron - located in the nucleus of the atom, it has an atomic mass unit (amu) weight of 1, it has no electrical charge, same size as a proton
Electron – located in orbit (or shell) around the nucleus, its weight is negligible, it has a negative electrical charge of minus (-) one
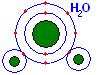
Compound = a combination of elements
Stable – Hard to break apart
Unstable – Easy to break apart
The mass of an atom is the combined ams of the protons and neutrons.
The number of protons = the number of electrons in a neutral atom.
An ion with one ore more electrons removed from or added to its outer orbit. It acts differently than an atom.
Sample problem:
An atom of mercury has an atomic number of 80 and a total mass of 200 amu.
What are the number of protons, neutrons, and electrons in this atom?
Protons = 80 (atomic number)
Electrons = 80 (electrons = protons in a neutral atom)
Neutrons = 120 (number of neutrons = atomic mass – number of protons)
Mass = 200 amu
Protons = 80 amu
Neutrons = 120 amu
Back to Homepage


